Endothelins are a group of strong vasoconstricting peptides. While vasoconstricting properties led to their discovery, endothelins are produced throughout the body and have receptors in virtually all cells. In addition to vascular effects, the endothelin system is intimately involved in a myriad of physiological processes including immunity, inflammation, embryonal development, growth, hypertrophy, fibrosis, and scarring.
Endothelin has 3 subtypes, numbered 1, 2, and 3. All are 21 amino acids in length and have similar structures, varying by several amino acids. The most powerful and clinically relevant subtype is endothelin-1 (ET-1). The others will not be discussed further in this review. There are two types of endothelin receptors, ETA and ETB, which are both clinically relevant and have different locations and effects.
The image below shows the balance of opposing effects of ETA and ETB on the vasculature. Endothelium-derived ET-1 binds to ETA and ETB receptors in vascular smooth muscle, causing contraction and increased vascular tone. At the same time, endothelium-derived ET-1 binds to ETB receptors in the endothelium itself, causing the release of PGI2 and nitric oxide, which act on nearby vascular smooth muscle cells, causing relaxation and decreased vascular tone.
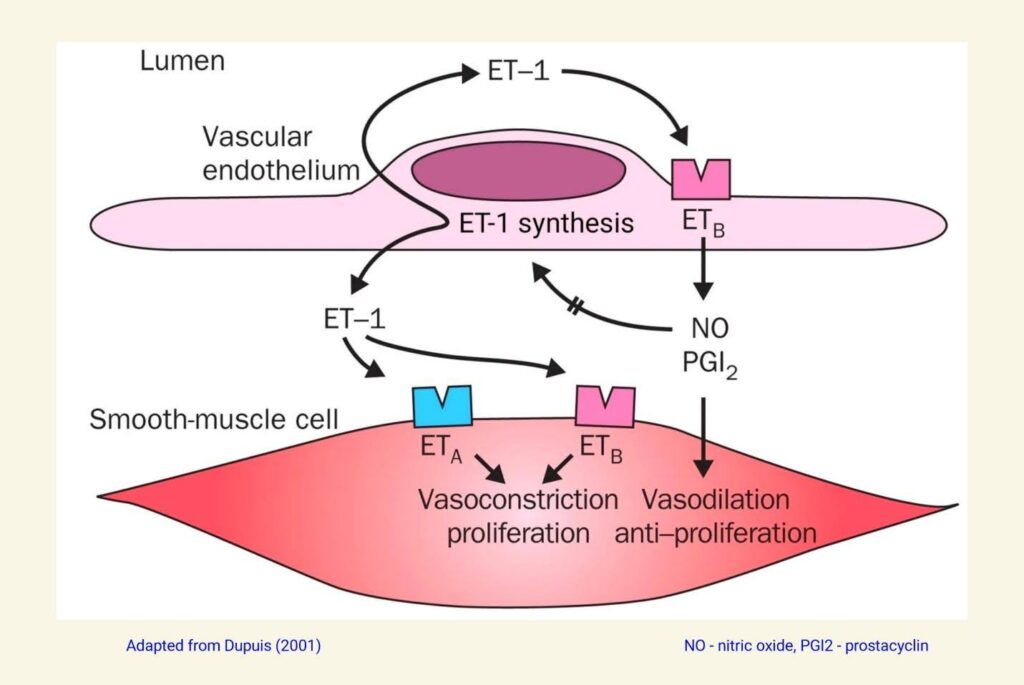
The balance between these opposing forces is determined by the specific vascular bed in an individual and of course, varies from person to person. This vascular endothelium-smooth muscle model also depicts autocrine (same cell type) and paracrine (nearby cell type) effects, which are recurrent features of endothelin physiology.
In 1991 the first orally active nonpeptide endothelin receptor antagonist (ERA), later named bosentan, was synthesized and became available for research. During the 1990s, more ERAs were synthesized and studied. ERAs that block both ETA and ETB receptors are classified as nonselective, and those that block primarily ETA receptors are classified as selective.
In 2001, bosentan (a nonselective ERA) was approved by the FDA for use in treating primary pulmonary artery hypertension. Since then, two other ERAs, ambrisentan (selective) and macitentan (nonselective) have been approved by the FDA for the same indication. Other than these three drugs, no new ERAs were approved for any indication until 2023.
Selective ETA receptor blockers have two theoretical advantages over nonselective ERAs. First, by not blocking ET-1 binding to endothelial ETB receptors, endothelial cells continue to produce nitric oxide and PGI2, which further promotes vascular smooth muscle relaxation and vasodilation. Second, in the distal nephron, ET-1 binds to ETB receptors in renal tubular cells, which blocks sodium and water resorption, causing a diuretic effect.
These theoretical advantages have not always translated into practical advantages. Numerous clinical trials have shown the most common side effects of both selective and nonselective ERAs to be sodium retention, volume overload, and edema, which seem to increase in incidence and severity in a dose-dependent manner. It appears that as systemic vasodilation increases, so do aldosterone levels, resulting in sodium retention.
Recent clinical trials have shown that with proper ERA dosing, problems with volume overload are greatly mitigated and usually manageable with diuretics when present.
With regards to renal disease, clinical trials have shown a general trend towards efficacy, with the caveat that many trials were stopped early because of the aforementioned problems with sodium retention. In 2023, the FDA approved sparsentan to reduce proteinuria in IgA nephropathy (IgAN) in selected patients. In 2024, this indication was expanded to add reducing decline in renal function in IgAN. Without getting too deep in the weeds in this review, IgAN is an autoimmune-mediated proteinuric glomerulopathy. Sparsentan is a dual endothelin angiotensin II receptor antagonist (DEARA), with endothelin antagonism selective for ETA receptors.
In addition to IgAN, ERAs have shown positive results in clinical trials of other proteinuric glomerulopathies such as diabetic nephropathy and focal segmental glomerulosclerosis (FSGS). Given the predisposition of ERAs to cause sodium retention and volume overload, clinical trials are ongoing that combine an ERA with an SGLT-2 inhibitor. The idea being that the diuretic effect of the SGLT-2 inhibitor might counteract the sodium avidity of the ERA, while the SGLT-2 inhibitor might also further treat the proteinuric glomerulopathy.
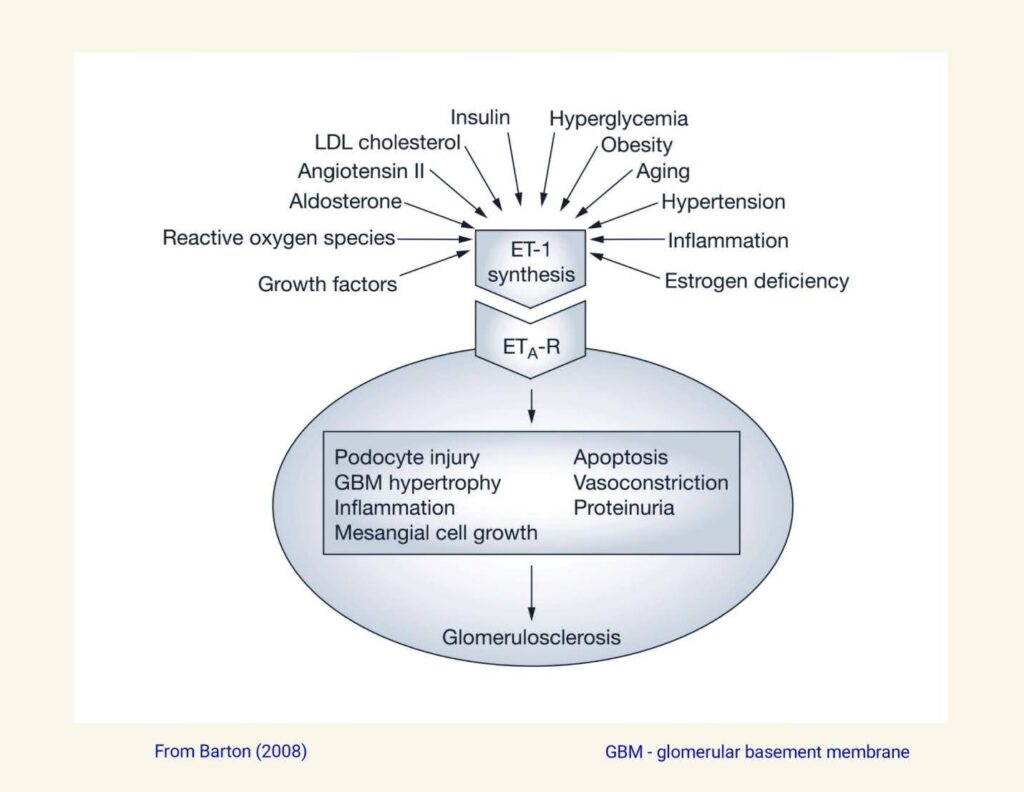
The image below shows some causes for elevated ET-1 levels in the kidney as well as various adverse consequences of elevated ET-1 levels.
In 2024, the ERA aprocitentan was approved by the FDA for treatment of resistant or complex systemic hypertension. Aprocitentan is considered a relatively nonselective ERA, with an ETA:ETB binding ratio of 16:1. ERAs like aprocitentan seem most effective as antihypertensives in low-renin, salt-sensitive patients or in patients where blockade of the renin-angiotensin-aldosterone axis with conventional antihypertensive medications has been unsuccessful.
Because of the possibility of embryofetal toxicity and the potential to cause major birth defects, ERAs must be used with great caution in females with reproductive potential. Because of this risk, all five commercially available ERAs can be prescribed and dispensed only by providers and pharmacies that participate in the manufacturers’ respective Risk Evaluation and Mitigation Strategy (REMS) programs.
References
- Barton M et al. (2019) Endothelin_30 Years from Discovery to Therapy Hypertension (74) p1232-65.
- Schiffrin EL, Pollock DM (2024) Endothelin System in Hypertension and Chronic Kidney Disease Hypertension (81) p691–701.
- Dupuis J (2001) Endothelin receptor antagonists in pulmonary hypertension Lancet (358) p1113-14.
- Barton M (2008) Reversal of proteinuric renal disease and the emerging role of endothelin Nature Clin Prac Neph 4(9) p490-501.
- Kohan DE, Barton M (2014) Endothelin antagonists in chronic kidney disease KI (86) p896-904.